Abstract
Introduction: Mutations (muts) in JAK2, MPL, and CALR are genetic hallmarks in myeloproliferative neoplasms such as myelofibrosis (MF). Prognostication in MF is predominantly based on clinical parameters according to the Dynamic International Prognostic Scoring System (DIPSS). However, gene mutations become increasingly important allowing for a more precised assessment of prognosis. For instance, CALR mutated MF is associated with favorable prognosis, while mutations in distinct high molecular-risk (HMR) genes are considered adverse. Our multicenter phase-Ib/II MPNSG-0212 trial (NCT01644110) investigating ruxolitinib plus pomalidomide in a total cohort of 92 patients with advanced MF and anemia provides an ideal basis for investigating the genomic landscape and molecular risk in a well-defined study population.
Aims & Methods: To assess the genomic landscape in MF patients treated within the MPNSG-0212 trial and to correlate the results with clinical parameters and overall survival (OS). So far, targeted next generation sequencing (NGS) of 269 candidate genes was performed in peripheral blood or bone marrow from 81/92 patients using libraries prepared with SureSelectXT HS (Agilent, Santa Clara, USA). NGS was carried out on a NextSeq550 (Illumina, San Diego, USA).
Results: At study entry, median age of the 81 patients was 71 years (range 52-86), median Hb 8.6 g/dL (range 5.4-11.7 g/dl); 30% of patients were RBC transfusion-dependent; 67% had primary MF (PMF) and 33% secondary MF (SMF), respectively. According to DIPSS, the vast majority of the patients were categorized as intermediate-2 (63%) or high-risk (26%) MF; 11% were low- and intermediate-1 risk patients.
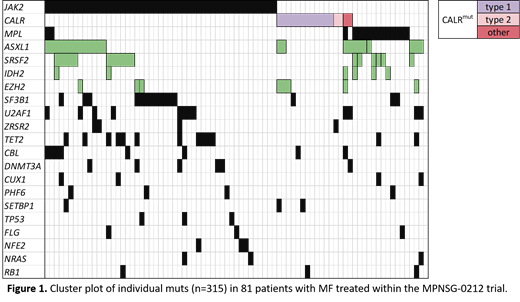
Overall, 315 muts were identified in 80/81 (99%) patients with a median of 3 muts/patient (range 0-9). Recurrent muts (≥5%) were identified in JAK2 (60%), ASXL1 (30%), SRSF2 (21%), CALR (20%; type-1: 75% [n=12], type-2 and non-type-1/2: 12.5% [n=2] each), MPL (19%), SF3B1 (19%), TET2 (16%), U2AF1 (15%), CBL and EZH2 (10% each), IDH2 and DNMT3A (7% each), PHF6, ZRSR2, and CUX1 (5% each).
The majority of the patients (95%) was characterized by the presence of a driver mut in JAK2, CALR, or MPL; 4/81 patients (5%) were triple negative (Figure 1). JAK2mut was associated with TET2mut (p=.047), whereas muts in CALR and TET2 were mutually exclusive (p=.05). CALRmut patients had less co-muts than patients with JAK2/MPL muts (mean 2.5 vs. 4.1, p=.007) and were mutually exclusive with muts in the spliceosome regulating genes SRSF2, SF3B1, U2AF1, and ZRSR2 (p=.009). Compared to MF with mutated JAK2 or MPL, MF patients with mutated CALR had a longer median OS (not reached vs. 3.1 years; p=.04).
With regard to high molecular risk (HMR) muts, n=56 were detected in 38 patients (47%), with 40% (15/38) of the patients harboring ≥2 HMR muts. The most commonly mutated HMR genes were ASXL1 (43%; 24/56), followed by SRSF2 (30%), EZH2 (14%), IDH2 (11%), and IDH1 (2%). MPLmut but not JAK2mut or CALRmut were significantly associated with HMR mut (p=.023). HMR mut patients harbored more co-muts than HMR wt patients (median 5 vs. 3; p<.0001). There were no significant differences in the variables age, sex, WBC, Hb, PLT, or LDH level between patients with HMR mut and HMR wt MF. In univariate analysis, patients with HMR mut MF had shorter median OS (2.3 vs 3.7 years, p=.007). In multivariate analysis (HMR mut, age, DIPSS-category, SMF vs. PMF) a higher DIPSS-score (HR, 3.2; 95% CI, 1.5-7.0; p=.004) and muts in HMR genes (HR, 3.5; 95% CI, 1.5-8.1; p=.003) were significant adverse prognostic factors for OS.
Conclusions: Our NGS data underline the genomic complexity of advanced MF. CALR mutations were only found in 20% of the patients that were characterized by less co-mutations, mutual exclusivity with spliceosome mutations, and with more favorable outcome suggesting a distinct disease biology. Almost 50% of patients showed mutations in HMR genes which were associated with an inferior OS in univariate and multivariate analyses.
§Frank Stegelmann and Konstanze Döhner contributed equally to this work.
Koschmieder: Shire: Honoraria, Other; Alexion: Other: Travel support; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: (e.g. travel support); Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: (e.g. travel support); Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: (e.g. travel support); Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: (e.g. travel support); Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: (e.g. travel support); Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: (e.g. travel support), Research Funding; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; AOP Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: (e.g. travel support), Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Baxalta: Membership on an entity's Board of Directors or advisory committees, Other; Abbvie: Other: Travel support; CTI: Membership on an entity's Board of Directors or advisory committees, Other; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: (e.g. travel support), Research Funding; Image Biosciences: Other: Travel support; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karthos: Other: Travel support. Heidel: Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AOP: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Hochhaus: Bristol-Myers Squibb: Research Funding; Pfizer: Research Funding; Incyte: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Hebart: AbbVie: Honoraria; AstraZeneca: Honoraria; BMS: Honoraria; Janssen: Honoraria; Roche: Honoraria. Isfort: Alexion: Other: Travel reimbursement; Roche: Other: Travel reimbursement; Amgen: Other: Travel reimbursement; Mundipharma: Other: Travel reimbursement; Hexal: Other: Travel reimbursement; BMS: Honoraria; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel reimbursement; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel reimbursement. Reiter: AOP Orphan Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; Deciphera: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Abbvie: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Blueprint Medicines: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding. Waller: Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Mylan: Consultancy; Alvotech: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees; Lilly: Membership on an entity's Board of Directors or advisory committees, Other: travel support; Chugai: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Other: travel support; Amgen: Membership on an entity's Board of Directors or advisory committees; IPSEN: Other: travel grant. Scheid: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Goethert: Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Travel support; Incyte: Consultancy, Honoraria, Other: Travel support; zr pharma&: Honoraria; BMS: Consultancy, Honoraria, Other: Travel support; AOP Orphan Pharmaceuticals: Honoraria, Other: travel support; Proteros Biostructures: Consultancy. Schafhausen: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Swedish Orphan Biovitrum AB: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees. Radsak: Otsuka: Consultancy, Honoraria; Abbvie: Other: e.g. travel support; Astellas: Other: e.g. travel support; TEVA: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: e.g. travel support; Daiichi Sankyo: Consultancy, Honoraria, Other: e.g. travel support; Celgene/BMS: Consultancy, Honoraria, Other: e.g. travel support; Amgen: Other: e.g. travel support; Takeda: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Corat: Consultancy, Honoraria; Cogent Biosciences: Consultancy, Honoraria; JAZZ: Other: e.g. travel support. Gattermann: Takeda: Research Funding; Novartis: Honoraria; Celgene: Honoraria. von Bubnoff: Novartis: Honoraria; Takeda: Honoraria. Brümmendorf: Bristol Myers: Research Funding; Janssen: Honoraria; Novartis: Honoraria, Patents & Royalties, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Repeat Diagnostics: Research Funding; Takepart Media: Honoraria. Döhner: Celgene: Honoraria, Research Funding; Agios: Honoraria, Research Funding; GEMoaB: Honoraria; Astex Pharmaceuticals: Honoraria; Astellas: Honoraria, Research Funding; Oxford Biomedica: Honoraria; Novartis: Honoraria, Research Funding; Jazz Pharmaceuticals: Honoraria, Research Funding; Janssen: Honoraria; Helsinn: Honoraria; Gilead: Honoraria; AstraZeneca: Honoraria; Abbvie: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Berlin-Chemie: Honoraria; Roche: Honoraria; Pfizer: Research Funding. Griesshammer: Amgen: Consultancy, Honoraria; AOP Orphan: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; CTI: Consultancy, Honoraria; Shire: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria. Stegelmann: BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Döhner: Abbvie: Consultancy, Honoraria; Janssen: Honoraria, Other: Advisory Board; Jazz Roche: Consultancy, Honoraria; Daiichi Sankyo: Honoraria, Other: Advisory Board; Astellas: Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Agios and Astex: Research Funding.
Pomalidomide was shown to be active in patients with myelofibrosis in particular in the treatment of anemia (Tefferi et al 2009, Begna et al 2011, Mesa et al 2010)
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal